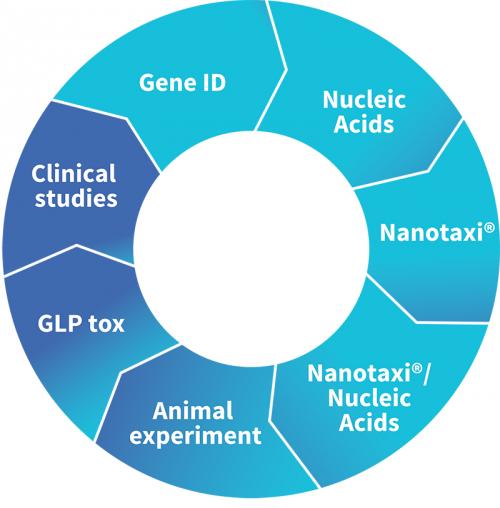
Technologies
Development process of the Nanotaxi®-based novel biotherapeutics
Target ID/Gene design
Identification of protein of therapeutic or antigenic interest
Design and optimization of the corresponding nucleic acid (DNA or mRNA) coding the proteins
Nucleic Acids Production
Production and quality control of plasmid DNA or mRNA encoding the protein of interest
Nanotaxi® production
Optimized In-Cell-Art proprietary Nanotaxi® produced by state of the art organic chemistry
Industrial synthesis of Nanotaxi® outsourced to GMP-approved CMO
Full package of chemical and physico-chemical characterization
Nanotaxi®/Nucleic Acids production
Supramolecular assemblies between Nanotaxi®/Nucleic acids produced by self assembling process. No use of specific mixing devices
Industrial fill and finishing of Nanotaxi®/Nucleic Acids outsourced to GMP-approved CMO
POC, animal experiment
Optimization of the protein of therapeutic or antigenic interest in the chosen animal specie including Non Human Primate
Optimization of the physiological activity (therapeutic or vaccination) in the chosen animal specie after Nanotaxi®/Nucleic acids administration
GLP tox
Safety profile of Nanotaxi® and Nanotaxi®/Nucleic acids assessed in safety toxicological studies
Injection of Nanotaxi® using conventional administration methods
Clinical studies
Clinical trials to assess safety and efficiency in humans
Projects
Main projects of research & development
-
Hepavac
- Preclinical development (efficiency, toxicity, bio-distribution) of a new immunotherapy vector composed of Nanotaxi® and plasmid DNA coding an anti- tumor antigen specific to hepatic cancer
€3.8m over 3 years (2010-2013)
-
RNArmorvax
- Objective is to develop and validate a new universal vaccine technology platform based on mRNA with substantial advantages over existing technologies
- Test this approach in prophylactic and therapeutic vaccines for infectious diseases
$33.1m over 4 years (2011-2015)
-
EMER-IT
- Accelerate the development of effective immunotherapy solutions against emerging health risk viruses with high pandemic potential (e.g. SARS, Ebola and Avian Flu H5N1)
- Prepare production and commercialization steps
€20m over 5 years (2012-2017)
-
EFFICACE
- Develop a new strategy of immunotherapy by producing with Nanotaxi/mRNA, antibodies involved in the control of immune reaction against tumor cells
- Evaluate the synergistic effects with the Hepavac vaccine to treat hepatocellular carcinoma